Aluminum¶
Introduction¶
Just like how the American Institute of Steel Construction developed the steel design code, the Aluminum Association (AA) is responsible for writing the Aluminum Design Manual. Aluminum is not commonly used in construction as major structural elements. It is, however, used in many construction assemblies and a lot of components that are exposed to the weather.
It is easier to discuss aluminum if you first understand the aluminum alloys' designations. The designations will consist of a letter (F, O, H, W, or T) that indicated the tempering (hover text) of the material. If H or T are used then they are followed by a number indicating the specifics of the treatment. There is also associated number, usually up to four digits long, that indicates the major alloying elements, modification of the alloy, and finally two digits that serve as an identifier.
| F | As fabricated and no mechanical properties specified |
| O | Annealed to obtain lowest strength temper |
| H | Strain-hardened wrought products or without additional thermal treatment. |
| W | Solution heat-treated (but subject to natural aging) |
| T | Thermally treated to produce a stable temper |
| 1 | Pure Aluminum |
| 2 | Copper |
| 3 | Manganese |
| 4 | Silicon |
| 5 | Magnesium |
| 6 | Magnesium and Silicon |
| 7 | Zinc |
| 8 | Other elements |
| T1 | Shaped hot and naturally aged |
| T2 | Shaped hot, worked cold and naturally aged |
| T3 | Solution heat treated*, cold worked and naturally aged |
| T4 | Solution heat treated and naturally aged |
| T5 | Shaped hot and artificially aged |
| T6 | Solution heat treated and artificially aged |
| T7 | Solution heat treated and artificially overaged |
| T8 | Solution heat treated, worked cold and artificially aged |
| T9 | Solution heat treated, artificially aged and worked cold |
| T10 | Shaped hot, worked cold and artificially aged |
| H1 | Strain hardened only |
| H2 | Strain hardened and partially annealed |
| H3 | Strain hardened, then stabilized |
| H4 | Strain hardened and lacquered or painted |
For example, 6063-T6 is an aluminum alloy that is combined with magnesium and silicon, with no modifications (indicated by the 0), then solution heat treated and artificially aged.
* Solution heat treatment is where very hot alloys are quenched in water "freezing" them, but allowing them to remain workable for a short time.
Here are a small series of background videos but they are not critical to view.
Sustainability¶
 In order to make aluminum sustainability all it take is a little recycling. Recycling aluminum only uses 5% of the energy needed to produce aluminum from bauxite ore. This results in a substantial reduction in greenhouse gases. Additionally, the majority of the aluminum in the world is produced using power from hydroelectric facilities. Though these have an environmental impact of their own, dams are considered a renewable source of power. Currently about 65% of aluminum is recycled in the United States and world wide 35% of all aluminum production comes from recycled material (source). Due to the long life span of most construction projects only about 8% of recycled aluminum is currently coming from construction salvage.
In order to make aluminum sustainability all it take is a little recycling. Recycling aluminum only uses 5% of the energy needed to produce aluminum from bauxite ore. This results in a substantial reduction in greenhouse gases. Additionally, the majority of the aluminum in the world is produced using power from hydroelectric facilities. Though these have an environmental impact of their own, dams are considered a renewable source of power. Currently about 65% of aluminum is recycled in the United States and world wide 35% of all aluminum production comes from recycled material (source). Due to the long life span of most construction projects only about 8% of recycled aluminum is currently coming from construction salvage.
Aluminum is used in a variety applications in the construction industry from lightweight, corrosion-resistant, panels to electrical wiring applications and extruded parts for windows. It also is used in exterior handrails due to durability.
Toxicity and the Environment¶
Aluminum is not generally toxic to anything. That being said, chromium metal may be present in some aluminum alloys, and hexavalent chromium, though not present in the alloy, may be formed during welding, and it is considered to be a carcinogen.
Also, like other powdered versions of construction material, it should not be inhaled or exposed to an open flame.
Source Aluminum alloys MSDS
Recycling¶
Most of what I have to say about recycling I've already said, except for aluminum is actually valuable enough that recyclers will pay for the scrap (around \$1 per pound as of June 19, 2015). That being said, if you are going to try to make money by recycling your scrap, it is important to secure it on the job site; it can be a target of thieves.
Reuse¶
 Aluminum products, like aluminum windows can be reused, but due to the less energy efficient nature of older windows, the reuse should be limited to unconditioned environments. Products like aluminum siding could be reused, but it is difficult to remove it in a reusable condition. Aluminum wire should not be reused if it has been removed from a structure. The reason for this is related to fatigue. Unlike steel, aluminum does not have a lower fatigue limit, and the cycling current in the wire creates enough physical stress in the wire to eventually lead to cracking and failure. The expand on this, not mater how big the piece of aluminum is, or how small the cycled load is, aluminum will eventually fail due to fatigue. That is not saying that it cannot be used effectively in construction, but its exposure to repeated loading should be considered when thinking about a particular application.
Aluminum products, like aluminum windows can be reused, but due to the less energy efficient nature of older windows, the reuse should be limited to unconditioned environments. Products like aluminum siding could be reused, but it is difficult to remove it in a reusable condition. Aluminum wire should not be reused if it has been removed from a structure. The reason for this is related to fatigue. Unlike steel, aluminum does not have a lower fatigue limit, and the cycling current in the wire creates enough physical stress in the wire to eventually lead to cracking and failure. The expand on this, not mater how big the piece of aluminum is, or how small the cycled load is, aluminum will eventually fail due to fatigue. That is not saying that it cannot be used effectively in construction, but its exposure to repeated loading should be considered when thinking about a particular application.
When examining a fatigue failure in aluminum, part of the surface will be dark where the crack was, and part of it will be bright where the final fracture occurred.
It is interesting to note that most commercial aircraft have aluminum skin, and aircraft aluminum skin has failed before due to fatigue. The loading cycle which causes failure, is due to the repeated pressurization and de-pressurization of the aircraft cabin. On a side note, in original airplane design the windows were not round, but rather they were square. This unfortunately lead to stress concentrations at the corners, which in turn lead to accelerated fatigue cracking. Surprisingly the method used to stop the propagation of fatigue cracking, is drilling a hole at the end of the crack. The round hole reduces the level of stress concentration at the tip of the crack, which in turn increases the fatigue lifespan of the remaining material.
The point of all this is that there are reasons that aluminum reuse is limited.
Material Properties¶
 Aluminum is both lightweight and ductile. It is also not magnetic (but is paramagnetic (video)) and, while in solid form, is unlikely to ignite. Its reflective surface does not easily tarnish, due to its propensity to quickly form a durable oxide coating. Aluminum has about one-third the density and stiffness of steel. In manufacturing can be machined, extruded, drawn or cast.
Aluminum is both lightweight and ductile. It is also not magnetic (but is paramagnetic (video)) and, while in solid form, is unlikely to ignite. Its reflective surface does not easily tarnish, due to its propensity to quickly form a durable oxide coating. Aluminum has about one-third the density and stiffness of steel. In manufacturing can be machined, extruded, drawn or cast.
Aluminum is both thermal and electrically conductor. However, is not nearly as conductive as copper, and it also has the tendency to break over time due to exposure to the stresses caused by fluctuating magnetic fields created by cycling current.
Strength¶
The aluminum alloy, 6063-T6, often used in handrails, has an ultimate tensile strength of at least 28 ksi, and yields at around 23 ksi. It is clearly weaker than steel, but it is also has a density of $168.5 \frac{lb}{ft^3}$ as apposed to steel's $490 \frac{lb}{ft^3}$, which is practically 3 times as much. This means, pound per pound, 6063-T6 aluminum is around as strong as grade 60 steel.
Some alloys of aluminum can be heat treated to a point where they yield at 100 ksi. Despite this aluminum is seldom used as major structural elements in buildings. The reasons for this can be cost or thermal compatibility, but one of the main concerns is fatigue.
One aspect of a material that effects its strength, is the method of connections used to join components. Aluminum is much like steel in its fastening characteristics. It can be both welded and bolted, but there are a few other techniques of fastening that are more common in aluminum than steel. These include riveting, stitching, interlocking joints, brazing, soldering, and adhesive bonding. Various versions of joints made using these techniques are commonly seen in ductwork on construction sites. The particular joint used depends on the specific application, and the resulting strength required of the joint. If a particular joint strength is required it may need to be verified, but the verification of the joint strength is, however, outside the scope of this class.
Structural Failure Modes¶

Environment Induced Failure Modes¶
 Aluminum can be damaged by repeated exposure to salt water, and it is also susceptible galvanic corrosion. Galvanic corrosion occurs when dissimilar metals are placed in contact, electrically, in the presents of an electrolyte. This can occur when aluminum touches stainless steel, copper, or titanium in the presents of water. Aluminum corrosion will appear as a white powder on the surface.
Aluminum can be damaged by repeated exposure to salt water, and it is also susceptible galvanic corrosion. Galvanic corrosion occurs when dissimilar metals are placed in contact, electrically, in the presents of an electrolyte. This can occur when aluminum touches stainless steel, copper, or titanium in the presents of water. Aluminum corrosion will appear as a white powder on the surface.
Chemical Weaknesses¶
Aluminum should not be placed in contact with strong acids. For instance HCl is one of the compounds that you must avoid having Aluminum exposed to. In addition to that, aluminum should not be placed in contact with strong bases. What happens is that the protective oxide coating degrades and dissolves. Then freshly exposed aluminum reacts with oxygen from the air, or ions from the base or acid (chloride ions in the case of HCl).
Chlorides should be avoided in general when dealing with aluminum because of its reactivity with them. If aluminum needs to be in placed contact with chloride salts, in concrete for instance, then the aluminum needs to be protected with some kind of coating or paint. It should be noted that chlorides are typically bad for regular concrete, and steel as well.
Video of aluminum reacting with both strong acids and bases.
Safe Operating Temperatures¶
Aluminum will begin to melt at around $1,200 ^\circ F$. However, it can begin to suffer damage by losing its protective oxide coating above $530 ^\circ F$. Once the oxide coating damaged, elemental aluminum will be directly exposed to oxygen. Aluminum is so highly reactive with oxygen, that it is actually used as a propellant in rocket fuel. The reason why we don't have aluminum cans exploding all the time is because the oxide coating will quickly reform, cutting off the oxygen from the bulk of the aluminum (its only when powdered is aluminum useful as rocket fuel). That being said, since there are better materials available for working at high temperatures, aluminum should only be used below $400 - 500 ^\circ F$.
Aluminum also expands nearly twice as much as steel when subjected to temperature fluctuations. This means that differential expansion between aluminum, and any connecting material to needs to be allowed for. This can be done with slotted holes for bolts, or laps in expansion joints. Which means this is easy enough to do during the design process, but it does need to be considered. If differential expansion isn't allowed for, then where ever pieces of aluminum are connected to more thermally stable elements, excessive stresses will build up, and will likely result in joint failure after only moderate changes in temperature.
Material Tests¶
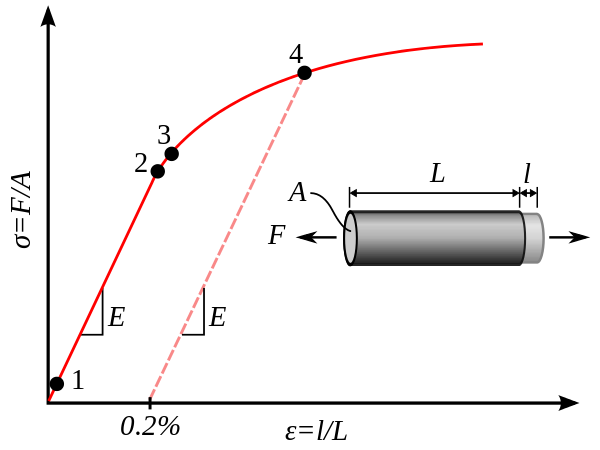 The main material test that is relevant for aluminum is, again, the tensile test. Other strength properties of aluminum are related to this value, and therefore many of those values can be derived from the yield, and ultimate tensile strength values. The curve you see to the right is qualitatively accurate for most aluminum alloys. The 0.2% offset you see is commonly used to find the yield strength in material that does not have a plastic region clearly defined in its stress-strain curve.
The main material test that is relevant for aluminum is, again, the tensile test. Other strength properties of aluminum are related to this value, and therefore many of those values can be derived from the yield, and ultimate tensile strength values. The curve you see to the right is qualitatively accurate for most aluminum alloys. The 0.2% offset you see is commonly used to find the yield strength in material that does not have a plastic region clearly defined in its stress-strain curve.
In order to calculate the 0.2% offset yield, a line is first drawn parallel to the elastic slope starting at a value of 0.2% strain. The yield stress is then defined as the value of stress found where the stress-strain curve intersects this offset line.
The Poisson's ratio of aluminum is seldom tested for since the values are readily available in tables for each of the alloys.
Quality Levels¶
There are many aluminum alloys, as previously indicated, but aluminum can also have many different types of finishes. These come in mechanically generated, chemically treated, and anodized varieties. These finishes are useful when achieving a particular architectural effect. The mechanical finishes are specified with a prefix of M, the chemical finishes are prefixed with the letter C, and anodic finishes are specified with an A prefix. The most important ones to remember are M10, C10, and A10 due to the fact that they indicate unspecified versions. If a different designation is indicated, then it will need to be verified before accepting delivery.
Listing of available finishes (pdf).
Residential Applications¶
Aluminum is used in the residential arena now days as cladding on steel sheet metal to provide corrosion resistance. You can still find it in existing homes used for window frames, but these are less common due to concerns over excessive thermal conductivity.
Aluminum is also used in any location where a corrosion resistant material would be useful, i.e. flashing, gutter systems, and screens. The value of this use case is that since it resists corrosion, it has an increased lifespan. This is particularly useful in applications that would be difficult to repair, or would be otherwise inaccessible. Valley flashing under asphalt shingles is a good example of this.
Commercial Applications¶
 Applications in commercial buildings, like with steel, are more numerous. Drop ceilings, duct work, and handrails are just a few of the additional locations that aluminum is used more frequently in commercial strictures vs. residential ones. The durable finishes and corrosion resistance make for an ideal maintenance free material.
Applications in commercial buildings, like with steel, are more numerous. Drop ceilings, duct work, and handrails are just a few of the additional locations that aluminum is used more frequently in commercial strictures vs. residential ones. The durable finishes and corrosion resistance make for an ideal maintenance free material.
Industrial Applications¶
 Aluminum is used in the industrial setting as more or less in a similar fashion as what is seen in both residential and commercial settings. One additional application though is the use of aluminum in high power lines. These lines are exposed to the weather, and thus need to be resistant to corrosion. They are still susceptible to fatigue, but are more readily accessible for inspection and repair.
Aluminum is used in the industrial setting as more or less in a similar fashion as what is seen in both residential and commercial settings. One additional application though is the use of aluminum in high power lines. These lines are exposed to the weather, and thus need to be resistant to corrosion. They are still susceptible to fatigue, but are more readily accessible for inspection and repair.
Cost Examples¶
Aluminum is sold in many shapes and sizes. It can come in the form of sheets, tubes, extruded parts, and nails among others. The prices of each of these are dependent on the raw material cost of aluminum, but are also strongly dependent on the working process needed to form the final product. There is not a good rule of thumb that can be used in estimating prices of aluminum products in this case. Just as a counter example you might think that aluminum flashing would be more expensive than steel flashing. On average you might find a roll of aluminum flashing (16"x50') for \$51.40, but that is not substantially more than the average price for the same size roll in steel, \$50.10. The reason the prices are so close is that less aluminum is necessary to achieve a comparable durability.
(Prices are estimated based on observed pricing trends on June 21, 2015)
Material Sources¶
Depending on the quantity and product you can purchase aluminum from commercial retailers, wholesalers, and manufacturers. This is such a generic list because of the wide variety of products that can be made out of aluminum. It is more instructive to consider sources based on products in this case rather than material.
Separate aluminum beams are likely only to be available form manufacturers and fabricators. Assemblies containing aluminum are likely only at retailers and wholesalers. Products like aluminum paneling, depending on order quantity, can be purchased from retailers, wholesalers, and manufacturers.
Examples of Installation Tools¶
You are more likely to personally install a product made from aluminum than steel, simply due to the lightweight nature of the material, and its ubiquity. To install an assembly that contains a substantial amount of aluminum you are likely to only need wrenches and/or screwdrivers. Assemblies containing aluminum are generally non-structural, and as such they will likely be fixtures, like wall mounted handrails, small cabinets, or light fixtures. You are less likely to personally be installing larger items, though you will have employees that might. The set of tools involved is mostly going to stay the same, though heavier tools might become needed. These might even include tools used for working with concrete, or even cranes if the weight is great enough.
Famous Failures¶
 One of the more famous instances of a failure of aluminum due to fatigue is that of Aloha Airlines Flight 234. The plane suffered explosive decompression when an 18 foot long section of the fuselage separated from the aircraft. The National Transportation Safety Board (NTSB) concluded that the accident was caused by metal fatigue exacerbated by crevice corrosion. The plane was operated in an environment where it was repeatedly exposed to salt and humidity. Additionally, a passenger boarding the plane noticed a crack in the fuselage, but failed to report it. Despite the substantial damage to the aircraft only one person died.
One of the more famous instances of a failure of aluminum due to fatigue is that of Aloha Airlines Flight 234. The plane suffered explosive decompression when an 18 foot long section of the fuselage separated from the aircraft. The National Transportation Safety Board (NTSB) concluded that the accident was caused by metal fatigue exacerbated by crevice corrosion. The plane was operated in an environment where it was repeatedly exposed to salt and humidity. Additionally, a passenger boarding the plane noticed a crack in the fuselage, but failed to report it. Despite the substantial damage to the aircraft only one person died.
This was not a construction failure, but I am including it since it is such a vivid example of failure due to fatigue in aluminum.
References¶
Github.io version of course website (Do not use this link if you are taking this course in Summer A or B.)
IPython.org (IPython is the opensource software used in the development of much of this course.)
Report any problems¶
Issues Please report any problems that occurred during the viewing of this or any other lecture.
Alternative Reporting - Email damontallen@gmail.com